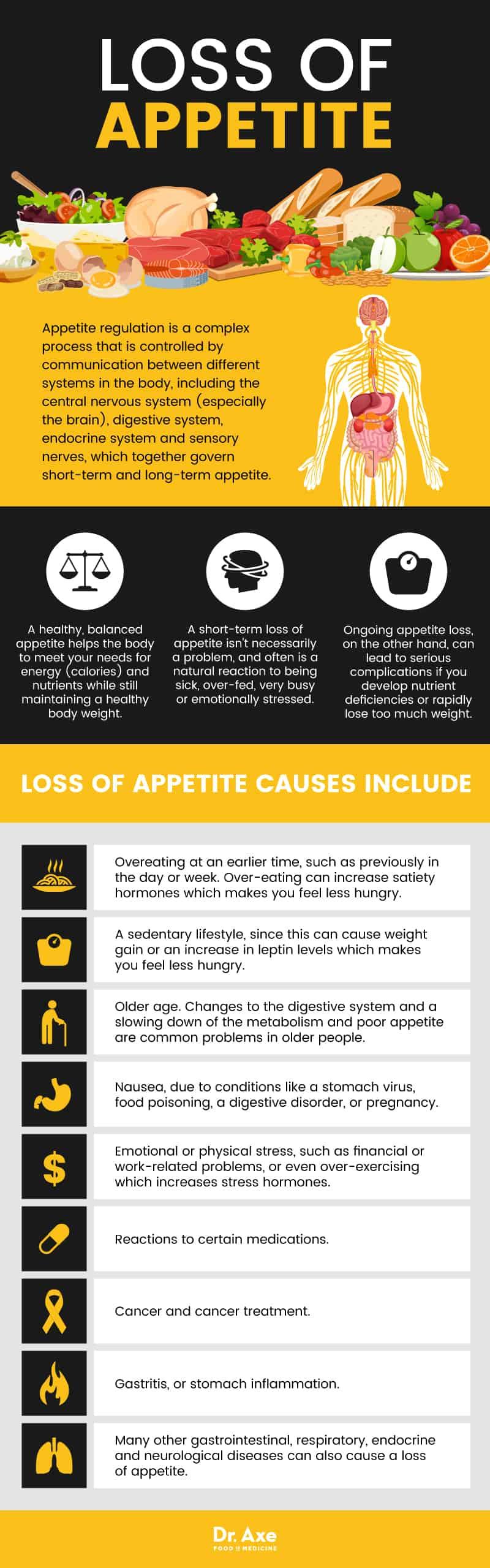
On March 26, 2025, the U.S. Food and Drug Administration (FDA) approved Vykat XR (diazoxide choline) extended-release tablets, developed by Soleno Therapeutics, as the first treatment for hyperphagia in individuals aged four and older with Prader-Willi syndrome (PWS). (sec.gov)
PWS is a rare genetic disorder affecting approximately 50,000 people in the United States. The hallmark symptom, hyperphagia, is characterized by an insatiable and persistent hunger, leading to significant weight gain and associated health complications. (reuters.com)
Vykat XR is a once-daily oral pill designed to target a specific pathway in the brain, reducing hyperphagia by decreasing the secretion of a peptide that regulates appetite. Clinical trials demonstrated that patients who remained on Vykat XR experienced a significant reduction in hyperphagia compared to those who switched to a placebo. (fiercepharma.com)
The approval of Vykat XR marks a significant milestone for Soleno Therapeutics, as it is the company’s first FDA-approved drug since its public listing in 2014. The company plans to make the drug available in the U.S. starting in April 2025. (sec.gov)
Wall Street analysts have responded positively to the approval, anticipating a strong launch for the drug. Stifel analyst James Condulis stated, "We think the drug’s well-positioned to launch strongly into what we see as a blockbuster opportunity." (fiercepharma.com)
The Prader-Willi Syndrome Association USA estimates that PWS occurs in one in every 15,000 live births. The approval of Vykat XR offers hope to many families affected by this challenging condition. (soleno.gcs-web.com)
For more information on Vykat XR and its availability, visit Soleno Therapeutics’ official website.
What other treatments are available for Prader-willi syndrome?
Table of Contents
Frequently Asked Questions (FAQ)
what is Prader-Willi syndrome (PWS)?
Prader-Willi syndrome is a rare genetic disorder affecting approximately 50,000 people in the United States. It is characterized by an insatiable and persistent hunger known as hyperphagia,leading to meaningful weight gain and associated health complications. ([reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-greenlights-first-ever-treatment-rare-metabolic-disorder-2025-03-26/?utm_source=openai))
what is Vykat XR?
Vykat XR (diazoxide choline) is a once-daily oral medication developed by Soleno Therapeutics. It is designed to reduce hyperphagia in individuals aged four and older with Prader-Willi syndrome by targeting a specific pathway in the brain that regulates appetite. ([reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-greenlights-first-ever-treatment-rare-metabolic-disorder-2025-03-26/?utm_source=openai))
How does Vykat XR work?
Vykat XR works by decreasing the secretion of a peptide that regulates appetite, thereby reducing the intense and persistent hunger associated with hyperphagia in Prader-Willi syndrome. ([reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-greenlights-first-ever-treatment-rare-metabolic-disorder-2025-03-26/?utm_source=openai))
What were the results of the clinical trials for Vykat XR?
Clinical trials demonstrated that patients who remained on Vykat XR experienced a significant reduction in hyperphagia compared to those who switched to a placebo. ([reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-greenlights-first-ever-treatment-rare-metabolic-disorder-2025-03-26/?utm_source=openai))
When will Vykat XR be available?
Vykat XR is expected to be available in the United States starting in April 2025. ([reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-greenlights-first-ever-treatment-rare-metabolic-disorder-2025-03-26/?utm_source=openai))
How much does Vykat XR cost?
The annual average cost of Vykat XR is $466,200, with dosing based on the patient’s weight.([reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-greenlights-first-ever-treatment-rare-metabolic-disorder-2025-03-26/?utm_source=openai))
How can I access Vykat XR?
For more data on accessing Vykat XR, visit Soleno Therapeutics’ official website or contact their patient support program, Soleno One, at 1-(833)-SOLENO-1 (1-833-765-3661) from 8 a.m. to 8 p.m. ET, Monday through Friday.
What is the importance of the FDA approval of Vykat XR?
The FDA’s approval of Vykat XR marks a significant milestone for Soleno Therapeutics and offers hope to many families affected by Prader-Willi syndrome, as it is the first treatment specifically approved to address hyperphagia in individuals with this condition. ([reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-greenlights-first-ever-treatment-rare-metabolic-disorder-2025-03-26/?utm_source=openai))